Case Study
Daniel A. Abreu, Gray O. Lovio, H. Zelenkova, J. Stracenska, Mr. Abreu, Pérez G. Orta
Melanil cream in the treatment of melasma at the hospital Manuel Fajardo (Cuba), 2009–2010
MELANILTM CREME IN DER MELASMABEHANDLUNG AM KRANKENHAUS MANUEL FAJARDO (KUBA), 2009–2010

Keywords | Summary | Correspondence | Literature
Keywords
hydroquinone., MelanilTM, melasma
Schlüsselworte
Hydrochinon., MelanilTM, Melasma
Summary
Background: Numerous therapeutic options have been tried in the treatment of melasma, but none is completely effective. Alternative therapy has proven to be an important option in medicine. MelanilTM is a product that combines as its main active ingredients Glycyrrhiza glabra, Uva ursi extract and Morus alba for melasma treatment. Objective: To evaluate the effectiveness of MelanilTM cream in the treatment of melasma. Method: Phase III open clinical trial, in two parallel groups, controlled and randomized at the dermatological service at the Hospital Universitario Clínico Quirúrgico Manuel Fajardo, Havana (Cuba), from October 2009 to October 2010. The patients were clinically diagnosed and by fluorescence, the sample being formed by 150 patients who met the inclusion criteria, two random groups of 75 each being established, treated topically with MelanilTM and hydroquinone 2%, respectively. Results: Patients were mainly between 40 and 49 years old, female, skin photo type III and suffered from the midface and epidermal type of melasma. Both groups largely showed favourable responses, with the MelanilTM group being the most effective according to the Melasma Area and Severity Index (MASI) and photo documentation, without the presence in this case of any adverse reactions. Conclusions: MelanilTM was effective in the treatment of melasma, with the advantage of being a formulation based on natural products with no adverse reactions.
Zusammenfassung
Hintergrund: Zahlreiche Behandlungsoptionen sind in der Therapie des Melasmas untersucht, jedoch ist kein hiervon hundertprozentig wirksam. Alternative Therapieverfahren haben sich zu einer wichtigen Option der Medizin entwickelt. MelanilTM ist ein Produkt, das folgende Substanzen als wesentliche Wirkstoffe kombiniert für die Behandlung des Melasmas: Glycyrrhiza glabra, Uva ursi-Extrakt und Morus alba. Ziel: Untersuchung der Wirksamkeit von MelanilTM Creme in der Melasma- Therapie. Methoden: Es handelt sich um eine offene Phase-III-Studie mit zwei Parallelgruppen, kontrolliert und randomisiert durch die dermatologische Abteilung am Hospital Universitario Clínico Quirúrgico Manuel Fajardo, Havana (Kuba). Sie wurde von Oktober 2009 bis Oktober 2010 durchgeführt. Die Diagnose erfolgte klinisch und mittels Fluoreszenz. Insgesamt erfüllten 150 Patienten die Einschlußkriterien. Es wurden zwei Gruppen von je 75 Patienten randomisiert, die entweder mit der MelanilTM Creme oder einer Creme mit 2% Hydrochinon behandelt wurden. Ergebnisse: Die Patienten waren überwiegend im Alter von 40 bis 49 Jahren, weiblich mit einem Hauttyp III und litten an einem zentrofazioalen epidermalen Typ des Melasmas. Beide Therapiegruppen erzielten positive Resultate, wobei sich MelanilTM gemessen am Melasma Area and Severity Index (MASI) und der Photodokumentation als wirksamer erwies. Zudem wurden keine unerwünschten Nebenwirkungen beobachtet. Schlussfolgerungen: MelanilTM Creme war in der Melasma-Therapie wirksam, zeigte keine unerwünschten Effekte und basiert auf natürlichen Inhaltsstoffen.
INTRODUCTION
Melasma, also known as chloasma or „pregnancy mask“, is an acquired symmetric, limited and irregular hypermelanosis characterized by light to dark brown patches in sun-exposed areas, especially on the face [1–7]. It is one of the most frequent causes of consultation in dermatology and the most common of all facial hyper pigmentation, primarily affecting women and although the incidence in Latino males is about 10%, a rate of 26% has been observed in India. Latinos, Asians and Orientals generally have a higher propensity to be affected [1, 3, 8–10].
Although its pathogenesis is unknown, there are many factors involved: endocrine, genetic predisposition, exposure to ultraviolet radiation (UVR), ingredients in some cosmetics, perfumes, medicines, metals, among others. It is thought that 50 to 70% of pregnant women may be affected and it is estimated that this condition may occur in 35% of women who use oral contraceptives [1, 2, 4–6, 11–14]. It is most frequently seen between the third and fourth decade of life, but sometimes earlier.
According to its topography it can be classified into: malar midface and mandibular. It can also occur with an extra facial location, as on the „V neck“ and upper limbs [1, 8, 15, 16]. The lesions may regress partially after pregnancy, when the treatment triggering them stops and by decreasing sun exposure, but they usually persist indefinitely. This condition does not cause any symptoms, but it does cause great psychological distress due to the marked aesthetic effect [15].
The diagnosis is primarily clinical, although a biopsy is performed if necessary. The Wood’s lamp examination exists as an auxiliary test, dividing it into four types: epidermal, dermal, mixed and indeterminate; the latter occurs in patients of skin types V and VI. Dermoscopy helps in the detection of underlying telangiectasia [17]. There are several methods that help to assess the severity quantitatively, but the MASI is one that allows us to determine the severity of the disorder more accurately and in a more systematic way [4, 15].
Historically, the main forms of treatment, which has been disappointing, include sunscreen, skin lightening, avoiding triggering or aggravating factors and patience, but currently there is no universally effective treatment. According to the consensus of the Grupo Mexicano para el Estudio de los Trastornos Pigmentarios (GMPETP), the most important therapeutic measure is photoprotection [4, 18, 19].
One of the main factors in achieving a satisfactory response is the adherence to treatment and to achieve a good doctorpatient relationship. If there is no change in habits, this will result in therapeutic failure, chronicity, relapse, multitreatment and, therefore, a recalcitrant melasma [4]. There are many skin lightening products, hydroquinone (HQ), which is the most widely used in the treatment of melasma, azelaic acid, kojic acid, vitamin C, niacinamide, flavonoids, phytic acid, grape juice extract and mulberry extract. According to its dosage form, they can be used in monotherapy (monodrug) or in combinations (double and triple), which aim to increase their lightening effect with the least possible adverse events [1, 4, 20, 21].
Many arrangements have been used, such as chemical peels, microdermabrasion, dermabrasion, laser, intense pulsed light therapy, etc. Any ablative procedure should be part of a comprehensive treatment with topical depigmenting agents and sunscreens, and is only indicated in patients who have not responded suitably to topical treatment alone [2, 4, 22–25].
Alternative therapy has proven to be an important option in medicine. Laboratorios Catalysis (Spain) have developed MelanilTM, a natural product that combines as its main active ingredients, amongst others, Glycyrrhiza Glabra, Uva Ursi extract and Morus Alba subjected to a molecular activation process that enhances its activity. The high frequency of the disease, which creates major psychological disorders in sufferers, being in a tropical climate where UVR play an important role in the pathogenesis of this disease and the complexity of a successful treatment for these patients have all led us to continue the search for another therapeutic option for the treatment of melasma.
METHOD
Patients
A Phase III open clinical trial was conducted, in two parallel groups, controlled and randomized, to assess the effectiveness of Melanil cream in patients with melasma. Patients from Havana were assessed. The research was conducted at the Hospital Universitario Clínico Quirúrgico Manuel Fajardo in the period between October 2009 and October 2010. It included 150 patients who met the following criteria:
Inclusion Criteria
- Clinical diagnosis and by fluorescence of melasma
- Being between 18 and 75 years old
- Signed informed consent
- Submitting skin phototypes I–IV
- Agreeing to meet photo protection measures Exclusion criteria
- Failure to meet any of the criteria for inclusion
- Patients taking steroids, oral contraceptives (containing estrogens and/or progesterone) and clofazimine who cannot stop this medication for the duration of the melasma treatment
- Patients using cosmetics that cannot stop using them during the research
- Patients at potential risk of not completing the study (those who are going to travel in the period of research, those living outside the city…)
- Pregnant women
- Women who are breastfeeding
- Use of hydroquinone or other topical medications three months before the start of the study
Ethics
The study was carried out pursuant to the principles established in the Declaration of Helsinki. It was approved by the Ethics Committee and the Scientific Board from the Hospital Universitario Clínico Quirúrgico Manuel Fajardo in Havana. All the patients signed the informed consent form agreeing to take part in the research programme. The clinical trial was registered on ClinicalTrials.gov (NCT0100162).
Organizing the clinical trial
After the initial examination, the patients who had satisfied the eligibility criteria were then included in the clinical study and randomized to one of the two groups made up of 75 patients each: The control group, patients who applied hydroquinone to 2%, and the experimental group, patients who applied MelanilTM (cream). Patients in both groups were instructed to apply the above 2 times a day in the affected area with a thin layer of cream after washing and drying the face, for a maximum of 8 weeks. All patients were instructed to take photo protection measures.
MelanilTM cream is produced by Laboratorios Catalysis, S.L. (Madrid, Spain). It is 100% natural. All its active ingredients, Glycyrrhiza glabra, Aspergillus ferment extract, Uva ursi extract, Morus alba and aloesin, have undergone a special process of molecular activation that enhances its activity. It comes in 50 ml bottles.
All patients were examined at the start, after 4 and 8 weeks.
The evaluation included a physical examination of the lesion; its specific characteristics and location being recorded in the medical history, thereby allowing us to specify the evolution of the lesion. The presence of any symptoms or signs that could be construed as an adverse reaction was investigated in each evaluation and photo documentation was performed.
Primary efficacy variable
Melasma Area and Severity Index (MASI): Its calculation expression is [6]: 4 areas were assessed: forehead + RCR (right cheek region) + LCR (left cheek region) + chin, with 30% each and 10% for the chin.
MASI = O.3A (D+H) + O.3A (D+H) + O.3A (D+H) + O.1A (D+H)
It is calculated by first assessing the hyper pigmentation area of the face. The melasma in each area (A) is given a numerical value: 1, < 10%; 2, 10–29%; 3, 30–49%; 4, 50–69%; 5, 70–89%; 6, 90–100%. The degree of darkness (D) in each area is assessed on a scale from 0 (absent) to 4 (severe). Homogeneity (H) is assessed on a scale from 0 (minimum) to 4 (maximum).
MASI is between 0 and 48, being mild when the result is between 0 and 11, moderate between 12 and 23, Severe between 24 and 36 and very severe between 37 and 48. The interpretation of the percentage obtained was as follows:
- MASI reduction between 0 and 24% = Poor.
- MASI reduction between 25 and 49% = Fair.
- MASI reduction between 50 and 74% = Good.
- MASI reduction of 75% and 100% = Excellent.
Secondary efficacy variable
Adverse reactions: Adverse reactions were described in the application of the product. Photo documentation: This was performed by 3 specialists in Dermatology, in the Excellent, Good, Fair and Poor categories by comparing the initial situation with the evolution after 4 and 8 weeks.
Statistical analysis
The baseline characteristics of the patients were summarized by means of absolute frequencies and percentages for the categorical variables. The Chi-square test was used to determine the homogeneity of the samples compared to the variables of interest. All the patients that had applied the cream at least once were included in the evaluation of the results (intention to treat analysis).
The evaluation of the response was summarized by means of absolute frequencies and percentages. The Chi-square test was used to determine the homogeneity of the samples compared to the primary and secondary efficacy variables. To investigate differences in the proportion of patients with mild melasma between groups, a test was used for comparing proportions from independent samples.
The safety analysis included all patients who self-administered the product at least once. The clinical trial was designed to include 150 patients. All the tests carried out were twotailed with a 5% significance level. The statistical analysis was carried out using SPSS Inc. for Windows, version 15, Chicago, IL.
RESULTS
The trial included 150 patients, 75 in each group. 21 patients (14%) dropped out, 9 in the MelanilTM group and 12 in the HQ 2% group. The causes of dropping out were: 4 patients (2.7%) for unforeseen travel, 4 (2.7%) for family problems, 3 (2.0%) for exacerbation of underlying chronic disease and 8 (5.3%) unknown. Included in this group were two patients who showed moderate adverse reactions that led to the discontinuation of the treatment, belonging to the group treated with HQ 2%, representing 1.3% of the total.
Table 1 shows the general characteristics of patients. The groups were comparable with regard to age, gender, skin phototype, melasma location, melasma type and duration of symptoms.
Primary efficacy
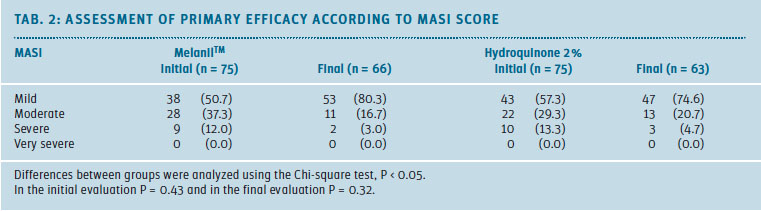
Table 2 shows the result of the primary efficacy evaluation. Melasma severity was assessed using the MASI score. At the beginning, the mild melasma was predominant in the sample, followed by the moderate and severe types. It was noted that the proportion of patients with moderate to severe melasma decreased towards the final evaluation.
After 8 weeks of applying MelanilTM (cream), 53 patients (80.3%) were in the mild category, an increase of 26.6% compared to the initial evaluation. In the HQ 2% group, 47 patients (74.6%) completed the study in the mild category, with an increase of 17.3% compared to the initial evaluation.
The difference in proportions between groups was not statistically significant (P = 0.57).
Secondary efficacy
No serious adverse events were reported. The adverse reactions were observed in the HQ 2% group. Two patients left the study due to moderate adverse reactions: Erythema, edema and desquamation that persisted for more than 48 hours. Minor adverse reactions that occurred were: Pruritus in 3 patients (4.8%), peeling in 3 patients (4.8%) and erythema and desquamation in 1 patient (1.6%)
The photo documentation analysis showed that 50 patients (75.8%) in the MelanilTM group had a favourable evolution, while in the HQ 2% group this figure was 43 (68.2%). (Tab. 3)
DISCUSSION
Melasma is most common in young adults. In the literature consulted, we found it was predominantly between 40 and 49 years old (similar to our study [12, 18]) and in others, between 30 and 39 years old, which was our second most frequent group [26, 23]. Sex, as in our work, is predominantly female in the literature consulted [9, 27, 28]. The skin types most affected are III to IV [14, 28]. If we compare our results, in which skin type III has also been predominant, we can see that they are consistent [14, 28]. The most widely represented location was the midface and the epidermal type, coinciding with an investigation conducted in 2010 [13], where 76% of patients had this pattern: midface, followed by the malar and only 1% with the mandibular type.
Other publications also demonstrate this [14, 26], in which this pattern held for 75, 63 and 50% respectively. In most of the literature consulted, the epidermis is the most common type of melasma [1, 4, 22]. Our results with relation to the duration of symptoms are consistent with several published works [26, 23]. The sample of this study was homogeneous in general.
In both treatment groups, mild melasma predominated, followed in descending order by the moderate and severe types. Our results are consistent with studies published in Europe and Asia, in which the highest percentage of patients had mild melasma at the start [14, 23]. Comparing our results with those found in our geographic area by other researchers [4], differences do exist because these have found a higher frequency of the moderate type. These discrepancies may be due to the fact that Cuban women have achieved a great level of responsibility in our society, carrying out important management roles, with a high scientific level and superior education, which encourages them to take immediate action at the onset of the disease.
Access to health services is not a problem either in our country, attending for treatment early when the condition is first apparent, a situation which is not shared by other countries, where differences were observed, despite the difficulty in complete resolution of this condition.
By analyzing the therapeutic response obtained solely by the criteria for the initial and final MASI, it can be seen that it was very similar in both treatment groups: patients with mild melasma increasing slightly in both groups and being slightly higher in the Melanil group. It is undisputed that there was a decrease in the MASI in both groups, which was not statistically significant, but it is clinically important because the number of patients who passed to the mild category increased, being slightly higher in the MelanilTM group. Our results are consistent with other recent studies [23–26].
Photographic documentation is a subjective variable, but important from a clinical point of view to compare results and graphically display the changes that have occurred in the lesion under study.
Another criterion of treatment response was the non-presence of severe adverse reactions. Two patients in the HQ 2% group had to discontinue treatment for moderate adverse reactions, in which erythema, edema and desquamation appeared and persisted for more than 48 hours. They did not complete the study and are not therefore included in this analysis.
Adverse reactions in patients who reached the end of treatment occurred in patients treated with HQ 2%. In patients treated with MelanilTM there were no adverse reactions. On consulting published literature, a high percentage of adverse reactions with hydroquinone cream was found [1–3, 16], their concentration being an important element to bear in mind. There are studies with Hydroquinone 2% type carried out by Sánchez et al in 2009 [18], where pruritus predominated and represented 20% of the total. In a study conducted in our country [27], adverse reactions with HQ 2% were also found in 13%, 2 patients showing severe adverse reactions.
Our results show the good tolerability and safety of Melanil cream.
CONCLUSIONS
Melanil was effective in the treatment of melasma, with the advantage of being a formulation based on natural products with no adverse reactions.
Korrespondenz-Adresse
Dr. Olaine R. Gray Lovio
Dermatology Specialist
Master’s Degree in Infectious diseases
Assistant Professor
Hospital Universitario
Clínico Quirúrgico Manuel Fajardo
Havana, Cuba
ogray@infomed.sld.cu
Literatur
1. Rigopoulos MD, Gregoriou S, Katsambas A (2007) Hiperpigmentation and melasma. J Cosmetic Dermatol 6: 195-200.
2. Memorias Simposio Actualización Terapéutica en el Tratamiento del Melasma. Galderma: 2004. CD ROM.
3. Roche JA (2003) Nuevas opciones en el tratamiento del melasma. Dermatología venezolana 41: N0. 3.
4. Consenso del Grupo Mexicano para el Estudio de los Trastornos Pigmentarios, 2006. http://www.dcmq.com.mx/num0702/concenso.html
5. Molinet Duarte I (1972–1973) Cloasma y Melanosis de Riehl. Estudio Clínico y Terapéutico. Hospital Docente Comandante Manuel Fajardo [Tesis de Especialista]. ISCM-H.
6. Urroz Cuadra CU, López Estrada KP. Respuesta clínica a tretinoina al 0.05 % e hidroquinona al 4 % versus fluocinolona acetónida al 0.01 %,hidroquinona al 4 % y tretinoina al 0.05 %, en el tratamiento del melasma, en el Centro Nacional de Dermatología Dr. Francisco Gómez Urcuyo, en el período comprendido de septiembre del 2006 a enero del 2007. [Tesis de Especialista]. UNIVERSIDAD NACIONAL AUTÓNOMA DE NICARAGUA. UNAN-MANAGUA.; MARZO 2007.
7. Manjiri DP, Parth P, Markowski T, McMichael AJ, Feeldman SR, Balkrishnan R (2007) Melasma and its impact on health-related quality of life in Hispanic women. J Dermatol 18: 5-9.
8. Bernal AP, Pérez MA, Camacho F (2001) Management of facial Hyperpigmentation. Am J Clin Dermatol 1: 261-268.
9. Pichardo R, Vallejos Q, Feeldman SR,Schulz MR, Verma A, Quandt, et al (2009) The prevalence of melasma and its association with quality of life in adult male Latino migrant workers. Int J Dermatol 48: 22-26.
10. Alfonso Morejón S (1983) Dermatosis más frecuentes de la cara. (Estudio de 400 pacientes). Hospital Militar Central Dr. Carlos J. Finlay [Tesis de Especialista]. ISCM-H.
11. Taylos A, Pawaskar M, Taylor SL (2008) Prevalence of pigmentary disorders and their impact in quality of life: A prospective cohort study. J Cosmet Dermatol 7: 164-168.
12. Ortonne JP, Arellano I, Bemeburg M, Celestari T, Chan H, Grimes P, et al (2009) A global survey of de role of ultraviolet radiation and hormonal influences in the development of melasma. J Eur Acad Dermatol Venereol 23: 1954-1962.
13. Guinot C, Cheffai S, Latreille J, Dhaoui MA, Youssef S, Jaber K et al (2010) Aggravating factors for melasma: a prospective study in 197 Tunisian patients. J Eur Acad Dermatol Venereol 24: 1060-1069.
14. Ghersetich I, Troiano M, Brazzini B, Arunachalam M, Lotti T (2010) Melasma: Treatment with 10 % tretinoin peeling mask. J Cosmet Dermatol 9: 117-121.
15. Fallabella FR, Chaparro JV, Barona MI, Domínguez LS (2009) Dermatología Fundamentos de Medicina. Séptima Edición 28: 182-184.
16. Freedberg I, Eisen A, Fitzpatrick T (2001) Dermatología en Medicina General. 5ta ed. Buenos Aires: EditorialMédica Panamericana pp 350-365.
17. Grin CM, Friedman KP, Grant-Kels JM (2002) Dermoscopy: a review. Dermatol Clin 20: 641-646.
18. Sánchez AT, Román AS, Olivera RP (2009) Efficacy of dioic acid compared with hydroquinone in the treatment of melasma. Int J Dermatol 48: 893-895.
19. Prignano F, Ortonne JP, Buggiani G, Lotti T (2007) Therapeutical approaches in melasma. Dermatol Clin 25: 337-342.
20. Ferrándiz FC (1996) Dermatología Clínica. Mosby / Doyma Libros 21: 203-205.
21. Du Vivier A (1999) Atlas de Dermatología Clínica. Mosby. Segunda Edición 25: 483-488.
22. Cestari P, Arellano I, Ortonne JP, Hexsel D (2009) Melasma in Latin América: options for therapy and treatment algorithm. J Eur Acad Dermatol Venereol 23: 760-772
23. Sharquie KE, Al-Mashhadani SA, Salman HA (2008) Topical 10% zinc sulfate solution for treatment of melasma. Dermatol Surg 34: 1346-1349.
24. Cestari TF, Hexsel D, Viegas ML (2007) Validation of melasma quality of life questionnaire for Brazilian Portuguese language: the MelasQoL-BP study and improvement of QoL of melasma patients after triple combination therapy. Br. J Dermatol 156 (Suppl 1): 13-20.
25. Zelenková H, Stracenska MD (2009) Hiperpigmentations and novel therapeutic possibilities. Firts results of the international multicentre study. Svidnik.
26. Zelenková H (2008). Ensayo piloto para verificar los efectos de la aplicación de la crema Melanil de catalysis en pacientes con melasma y otras pigmentaciones. Resultados iniciales. Svidnik.
27. Mouris ON (1998) Resultados hipopigmentantes de la crema callibelle en condiciones tropicales. (Estudio de 100 pacientes). Hospital Hermanos Ameijeiras [Tesis de Especialista]. ISCM-H.
28. Cestari TF, Hassun K, Sittart A, Viegas ML (2007) A comparison of triple combination cream and hydroquinone 4 % cream for the treatment of moderate to severe facial melasma. J Cosmet Dermatol 6: 36-39.